
|
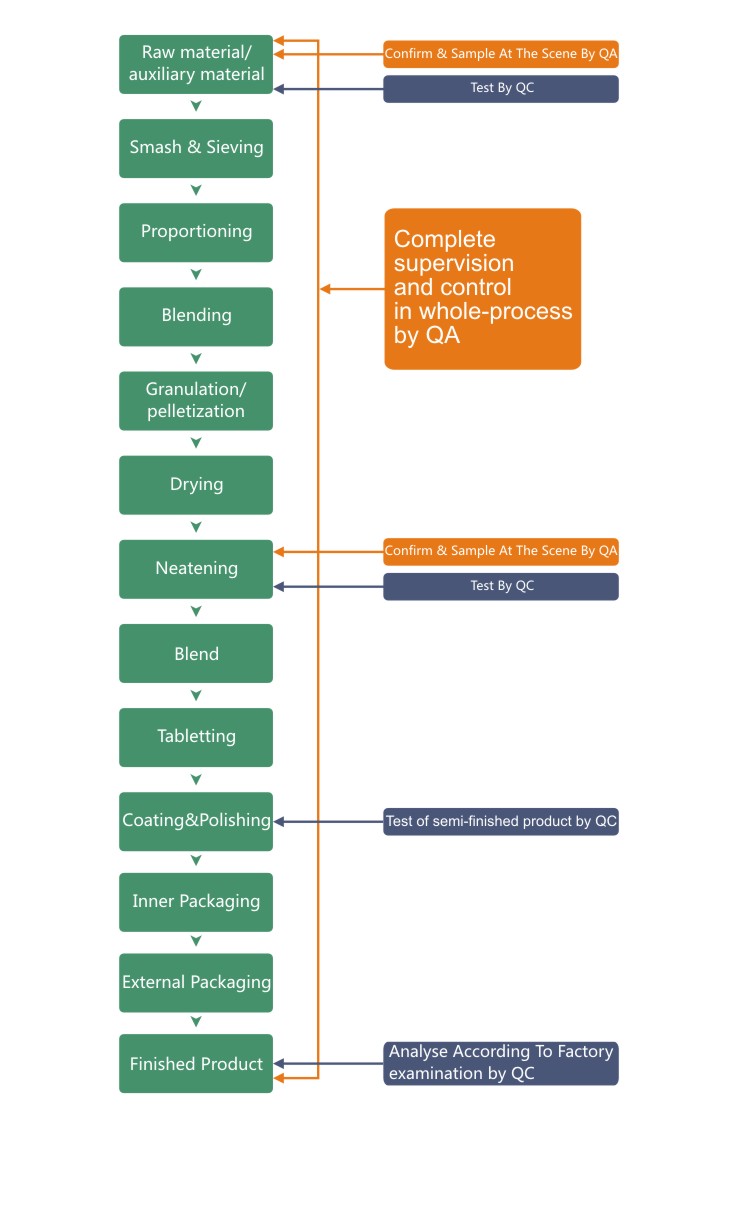
Safety and effective active ingredients in plants are reserved from the collection of starting material, griding and extraction to finished products. Proven quality management system ensures strict compliance with GMP requirements at each procedure. A detailed and complete record is maintained and documented appropriately for each batch of products to ensure steady quality.
Quality management System
|


 Shaanxi Provincial Food and DrugAdministration Party Secretary, Secretary Hu Xiaoping visited Mori Da...
Shaanxi Provincial Food and DrugAdministration Party Secretary, Secretary Hu Xiaoping visited Mori Da...
 Sciphar products show on 2017 FIC China International Food Additives and Ingredients Exhibition March...
Sciphar products show on 2017 FIC China International Food Additives and Ingredients Exhibition March...
 Shaanxi Sciphar health products show in the 77th Wuhan API Nov. 16th, the 77th China International Ph...
Shaanxi Sciphar health products show in the 77th Wuhan API Nov. 16th, the 77th China International Ph...
 Shaanxi Provincial Department of Commerce Director visit Sciphar Health Industrial Park. Shaanxi Prov...
Shaanxi Provincial Department of Commerce Director visit Sciphar Health Industrial Park. Shaanxi Prov...
 Shaanxi Sciphar in the enterprise development at the same time, in-depth development of industrial pr...
Shaanxi Sciphar in the enterprise development at the same time, in-depth development of industrial pr...
 Nov. 16th, the 77th China International Pharmaceutical Raw Materials, Intermediates, Packaging, Equip...
Nov. 16th, the 77th China International Pharmaceutical Raw Materials, Intermediates, Packaging, Equip...
 2017 "Sciphar Cup" China Central Ring Qinling Bicycle League stay in the station at the Purple Mounta...
2017 "Sciphar Cup" China Central Ring Qinling Bicycle League stay in the station at the Purple Mounta...
 Shaanxi Sciphar participated 2017 Vitafoods Asia Exhibition in Singapore
September 5 - 6, 2017 Vi...
Shaanxi Sciphar participated 2017 Vitafoods Asia Exhibition in Singapore
September 5 - 6, 2017 Vi...











